

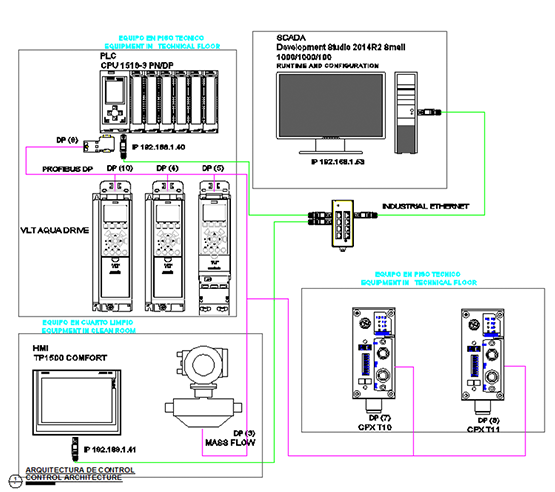
• Control Platform
• SIMATIC WIN CC 7.2
• SIMATIC LOGON
• S7-1500
The PLC system underwent factory acceptance testing by the client before delivery. These tests revealed inaccuracies in the panel and the control program's operating design. Working within a tight schedule before delivery and execution, Grupo SIEO engineers redesigned the panel drawings and modified the program to reflect a functioning system. The client was pleased because Grupo SIEO had the experience to develop a functional system, and potential startup issues were mitigated during the testing phase. Once engineering was complete, and panels were delivered, the installation task commenced and met project requirements and schedule for timely and successful project control. The delivered system was qualified for FDA audit, passing the audit without the need for subsequent modifications.
The project was designed and developed to comply with the following regulations:
EU CFR-21 (Code of Federal Regulations), Parts 210 and 211 (Good Manufacturing Practices, April 2007), Part 11 (Electronic Signatures and Records, August 2001), and Part 820 (Software Validation and Automated Equipment, April 2007).
GAMP4 Guide for Validation of Automated Systems, December 2001, Published by ISPE.
General Principles of Software Validation; Final Guidance for Industry and FDA Staff, January 2002.