
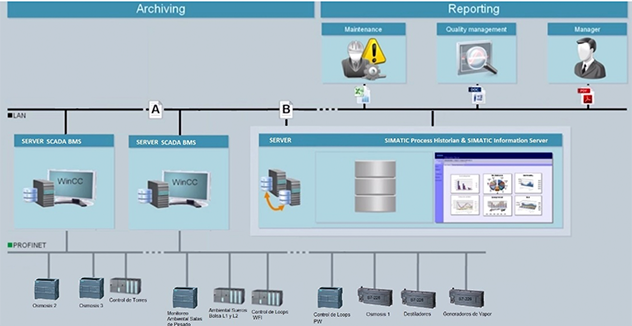
• Simatic WIN CC Server 7.2
• Simatic Logon
• Simatic Process Historian
• Simatic Information Server
Operators, quality personnel, validation, and maintenance staff are more than satisfied with the results.
"Reports are generated with a lot of detail and obtained much more quickly."
It is easier to find data without having to jump from application to application.
"Now the systems are monitored with greater reliability"
The project was designed and developed to comply with the following regulations:
EU CFR-21 (Code of Federal Regulations), Parts 210 and 211 (Good Manufacturing Practices, April 2007), Part 11 (Electronic Signatures and Records, August 2001), and Part 820 (Software Validation and Automated Equipment, April 2007).
GAMP4 Guide for Validation of Automated Systems, December 2001, Published by ISPE.
General Principles of Software Validation; Final Guidance for Industry and FDA Staff, January 2002.