

• Interconnection
• Commissioning
• Documentation
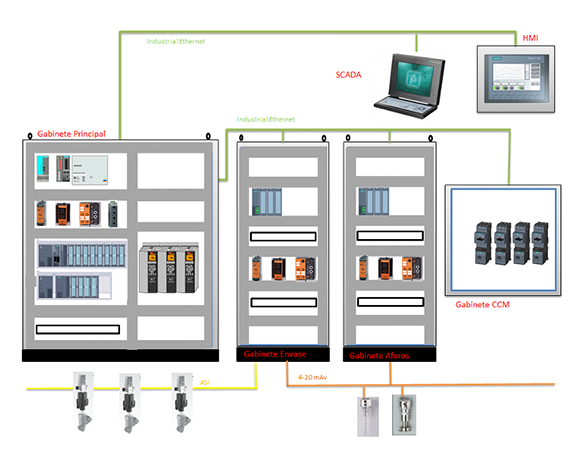
The Grupo SIEO team carried out the construction of electrical conduits, wiring, installation and interconnection of sensors, assembly of control cabinets, commissioning of automatic systems controlling the manufacturing and packaging process, documentation, and qualification of a line that produces injectable solutions in bags. The project's success was achieved through the special training and experience of the team in pharmaceutical work. The expertise and training of the engineers in charge ensured that construction, commissioning, and system startup were completed on time. Some client feedback upon completion was, "SIEO's technical staff is highly trained; we will request their involvement during the construction and commissioning of future lines."
The project was designed and developed to comply with the following regulations:
EU CFR-21 (Code of Federal Regulations), Parts 210 and 211 (Good Manufacturing Practices, April 2007), Part 11 (Electronic Signatures and Records, August 2001), and Part 820 (Software Validation and Automated Equipment, April 2007).
GAMP5 Guide for Validation of Automated Systems, December 2010, Published by ISPE.
General Principles of Software Validation; Final Guidance for Industry and FDA